By Laurie McGinley, The Washington Post.
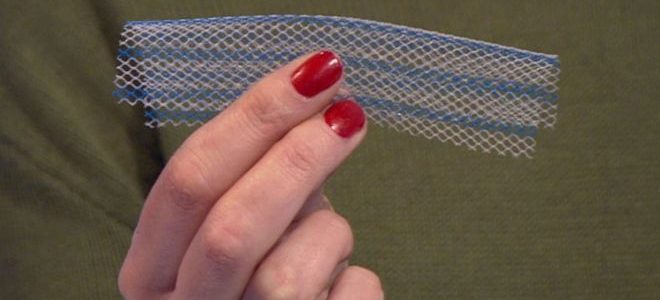
The Food and Drug Administration on Tuesday ordered manufacturers of surgical mesh used for repairing a condition called pelvic organ prolapse to immediately stop selling their products, the latest turn in a years-long battle over the safety of the implants.
The agency said the companies, Boston Scientific and Coloplast, had not demonstrated a “reasonable assurance” of safety and effectiveness for the devices for use over the long term, and will have 10 days to submit plans for withdrawing them from the market.
The FDA action specifically affects surgical mesh used for the transvaginal repair of pelvic organ prolapse, which occurs when the muscles and tissues supporting the uterus, bladder or rectum become weak or loose. That can allow organs to drop or press into the vagina. The regulatory action does not apply to mesh used for other conditions, such as hernias or stress urinary incontinence.
The FDA move, its toughest action yet against the devices, comes after tens of thousands of women have filed lawsuits against mesh manufacturers claiming injuries including bleeding and pain. The agency said there has been an increase in reports of adverse events in the last several years involving the devices.
In 2016, the FDA reclassified the products as high-risk devices — subjecting them to the agency’s most stringent path for device oversight — and required manufacturers to obtain approval to continue to market them. Many manufacturers withdrew from the market amid the heightened regulatory scrutiny and the growing number of lawsuits.
“In order for these mesh devices to stay on the market, we determined that we needed evidence that they worked better than surgery without the use of mesh to repair pelvic organ prolapse,” said Jeffrey Shuren, director of the agency’s Center for Devices and Radiological Health. “That evidence was lacking in these premarket applications, and we couldn’t assure women that these devices were safe and effective long term.”
Public Citizen, which first petitioned the FDA in 2011 to ban the products, welcomed the move, but said it came “too late for the thousands of women who have been irreparably harmed by these devices.” The group said it has been clear for several years that the products “are unsafe for treating pelvic organ prolapse and lack any clinically significant benefits in comparison to nonmesh products.”
Boston Scientific said in a statement that the company was “deeply disappointed” by the agency’s decision, adding that “patient safety is always our highest priority.” The company said the FDA action will “severely limit” options for women seeking treatment for pelvic organ prolapse.
Coloplast declined to comment.
The FDA said that surgeons began using surgical mesh to repair abdominal hernias beginning in the 1950s. In the 1970s, gynecologists began implanting surgical mesh for abdominal repair of pelvic organ prolapse and two decades later for the transvaginal repair of the condition, the FDA said. In 2002, the first mesh device for transvaginal repair of pelvic organ prolapse was cleared by the agency as a device that carried “moderate risk.”
About 1 in 8 women have surgery to repair the condition, and a subset of the procedures use the mesh, the agency said. It added that the proportion of women undergoing the mesh procedures decreased after the FDA began issuing warnings.
The agency said the women who have had transvaginal mesh used for their condition don’t need to take any action if they are satisfied with their surgeries and don’t have any symptoms. But they should notify their physicians if they have vaginal bleeding or discharge, pelvic or groin pain or pain during sex, the agency said.
Apr 16, 2019